Product Description
We are enable to supply two type of entecavir: mirconized power/common power.
Entecavir monohydrate(209216-23-9) is a nucleoside analog(more specifically, a guanine analogue) that inhibits reverse transcription, DNA replication and transcription in the viral replication process.
Thera. Category: Adult chronic hepatitis B
Cas No.:209216-23-9
Synonym:entecavir hydrate;ENTECAVIR MONOHYDRATE;2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate;Entecavir hydrate / 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate
Molecular Formula:C12H17N5O4
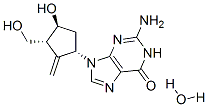
Molecular Weight:295.29448
Pharmacopeia: In house Spec.
Specifications:Available on request
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Usage:This product is applicable to the treatment of adult chronic hepatitis B.
CERTIFICATE OF ANALYSIS
|
Product Name
|
Entecavir Monohydrate
|
Cas No.
|
209216-23-9
|
|
Producing Date
|
2014-10-03
|
Batch No.
|
20141003
|
|
Assaying Date
|
2014-10-03
|
Reporting Date
|
2014-10-03
|
|
Expiry Date
|
2016-10
|
Quantity
|
50Gram
|
|
Items
|
Specifications
|
Results
|
|
Appearance
|
White or off-white powder
|
White powder
|
|
Identification
|
1)The IR absorption specturm of the sample must be same with entecavir
2) RT of the sample should match that of standard assay preparation
|
Comform
|
|
Water
|
5.8%~6.5%
|
6.1%
|
|
Optical rotation
|
+24°~+28° (DMF:MOH=1:1 C=1%)
|
+27.2°
|
|
Residue on ignition
|
≤0.10%
|
0.07%
|
|
Heavy Metals
|
≤20ppm
|
Conforms
|
|
Related substances
|
Total impurities
|
≤0.30%
|
0.07%
|
|
Single impurity
|
≤ 0.1%
|
0.03%
|
|
Residual solvents
|
dichloromethane
|
≤600ppm
|
100PPM
|
|
methanol
|
≤1000ppm
|
300PPM
|
|
tetrahydrofuran
|
≤700ppm
|
ND
|
|
toluene
|
≤600ppm
|
ND
|
|
Assay (HPLC)
|
99.8% ~102.0%(On anhydrous basis)
|
≥99.9%
|
|
Particle size
|
D90≤30um
|
Conform
|
|
Conclusion: The product corresponds to the requirements of in house standard.
|
|
|
Related intermediates:
1)2-Amino-6-Benzyloxypurine 19916-73-5
2)(1S,2R,3S,5R)-3-(Phenymethyloxy)-2-(phenylmethoxy)methyl-6-oxabicyclo[3.1.0]hexane 110567-22-1
3)(1S,2S,3S,5S)-5-(2-Amino-6-(benzyloxy)-9H-purin-9-yl)-3-(benzyloxy)-2-(benzyloxymethyl)cyclopentanol 142217-77-4
4)(2R,3S,5S)-3-(Benzyloxy)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-2-(benzyloxymethyl)cyclopentanol 142217-78-5
5)2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one CAS 142217-81-0

.png) Contact Now
Contact Now
