Product Description
Palbociclib (OTAVA-BB 1115529) CAS number is 571190-30-2 ,it is an inhibitor of cyclin-dependent kinases (CDKs) 4 and CDK6. Cyclin D1 and CDK4 / 6 are downstream of the cell proliferation signaling pathway. In vitro, Palbociclib reduces cell proliferation in estrogen receptor (ER) -positive breast cancer cell lines by blocking the progression of cells into the S phase from the cell cycle G1. Compare the combination of Palbociclib and anti-estrogen drug and administration of each drug alone,it can reduce retinoblastoma protein (Rb) phosphorylation resulting in E2F expression and prevent an increase in growth. In vitro treatment of ER-positive breast cancer cell lines with both Palbociclib and antiestrogens leads to an increase in cellular senescence, which can persist for 6 days after drug removal. In vivo studies using a patient-isolated ER-positive breast xenograft animal model showed that the combination of paclitaxel and letrozole alone increased the inhibition of Rb phosphorylation, downstream signaling and tumor growth compared to individual drugs . on February 3, 2015, FDA Accelerated Approval of Pfizer Palbociclib CAS 571190-30-2 in the Form of Capsules Available in 75 mg, 100 mg and 125 mg Formulations.Palbociclib (OTAVA-BB 1115529) CAS 571190-30-2 in combination with Letrozole as an initial endocrine therapy-based regimen for the treatment of postmenopausal women with estrogen receptor (ER) -positive, human epidermal growth factor receptor 2 (HER2) -negative postmenopausal women with advanced breast cancer .
Thera. Category: Vasopressin Receptor
Cas No.: 571190-30-2
Synonym:Palbociclib API;palbociclib (OTAVA-BB 1115529); Palbociclib, >=98%;tert-butyl 4-(6-(8-cyclopentyl-5-Methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyrido[2,3-d]pyriMidin-2-ylaMino)pyridin-3-yl)piperazine-1-carboxylate (CAS No.866084-31-3);Prabhu past Lieb;6-Acetyl-8-cyclopentyl-5-methyl-2-{[5-(1-piperazinyl)-2-pyridinyl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one
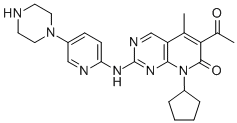
Molecular Weight: 447.54
Molecular formula: C24H29N7O2
Assay: ≥98.%
Appearance: White Crystalline solid
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
