Product Description
Valecoxib cas number 181695-72-7, the appearance of white crystalline powder. It is a nonsteroidal antiinflammatory drug with antiinflammatory, analgesic and antipyretic effects by inhibiting cyclooxygenase-2 (COX-2),then inhibiting the synthesis of prostaglandins . Clinically used to treat to relieve arthritis, adult rheumatoid arthritis, primary dysmenorrhea, postoperative pain, etc., each of the different symptoms are not recommended dose. The oral bioavailability of Valecoxib is about 80%, mainly through liver cytochrome metabolism, and about 70% of the metabolites are excreted with urine. The main adverse reactions were indigestion and gastric ulcer, but the incidence was lower than that of naproxen. Allergy with aspirin, nonsteroidal anti-inflammatory drugs, sulfonamides drug are prohibited. In 2004, the US FDA published a new adverse effect of Valecoxib cas number 181695-72-7,valecoxib may increase the risk of fatal skin adverse reactions and warn that postoperative application of daroxacin increases the incidence of cardiovascular events.
Thera. Category: Rheumatoid Arthritis
Cas No.: 181695-72-7
Synonym:Bextra, 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenefulfonamide;4-(5-methyl-3-phenyl-oxazol-4-yl)benzenesulfonamide;Bextra, 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide;4-(5-METHYL-3-PHENYL-4-ISOXAZOLYL)BENZENESULFONAMIDE;AKOS 92130;BEXTRA;VALDECOXIB;BEXTRA(VALDECOXIB);
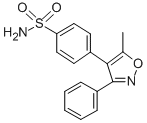
Molecular Formula:C16H14N2O3S
Molecular Weight: 314.36
Assay: ≥99.%
Packing: Export worthy packing
lMaterial Safety Data Sheet: Available on request
Usage: Synthesis Pharmaceutical Intermediates
Related intermediate:
1) 5-Methyl-3,4-diphenylisoxazole cas number 37928-17-9
2) 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonyl chloride CAS number 509074-26-4
3) Valecoxib cas number 81695-72-7,

.png) Contact Now
Contact Now
