Product Description
ABT199 intermediate 4,cas number is 1628047-84-6, synthesis process is as following:
First step: In a 100 ml three-necked flask, 50 mL of N,N-dimethylformamide and 11.0 g of 5-bromo-7-azaindole were added, and the mixture was stirred and dissolved, and then cooled to 0℃. Then, 2.45 g of sodium hydride was added in portions, and the mixture was stirred at 0 to 5℃ for 0.5 h, then 9.6 g of p-methoxybenzyl chloride PMB-Cl was added dropwise, and the mixture was naturally added to room temperature for 2 h. TLC is controlled and the reaction is over. The reaction solution was slowly poured into 200 ml of water, and extracted with 50 ml of EA three times. The organic phase was combined, washed three times with 100 ml of water, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain the first step compound 14.6gram 1 -(4-Methoxybenzyl)-5-bromo-7-azaindole.
Step 2: Add 7.1 g of the first step compound, 117 mg of copper acetylacetonate, ligand N, N'-bis(4-hydroxy-2,6-dimethylphenyl) grass to a 100 ml three-necked flask at room temperature. 147mg of Amide (BHMPO), 1.97 g of lithium hydroxide monohydrate, 28 ml of dimethyl sulfoxide, 7 ml of water, stirring was started, and the temperature was raised to 100℃ under nitrogen atmosphere, and the temperature was maintained for 8 hours. TLC controlled raw material reaction was completed, the heating was stopped, the temperature was lowered to room temperature, 210 ml of water was added, and the pH was adjusted to 6 with 1 N dilute hydrochloric acid. After solid precipitation, it was cooled to 0-5℃, stirred for 2 h, and then filtered with suction. The mixture was rinsed twice and dried at 50 ° C to give 5.4 g of 1-(4-methoxybenzyl)-5-hydroxy-7-azaindole.
The third step:1.0gram of the second step compound 1.0g, 0.88gram of methyl 2,4-difluorobenzoate was added to 10mL diethylene glycol dimethyl ether, then added 1.67g anhydrous potassium phosphate, under nitrogen protection 110℃, The reaction was overnight. The reaction solution was poured into 100 mL of water, extracted three times with 30 mL of EA, and the organic phase was dried over anhydrous sodium sulfate and dried to give the compound of the third step 2-((1-(4-methoxybenzyl)-1H-pyrrole. [2,3-b]pyridin-5-yl)oxy)-4-fluoro-benzoic acid methyl ester 1.2 g.
Step 4: Under nitrogen protection, add 1.0 g of the third step compound and 5 ml of dichloromethane to a 50 ml three-necked flask, stir and dissolve, cool to -45℃, and add a 1.0 M solution of boron trichloride in dichloromethane 15ml, the temperature is controlled at -45 ~ -35℃. After 1 h of incubation, the mixture was slowly warmed to room temperature overnight. The TLC controlled raw material reaction is over. The reaction liquid was added to 10 ml of methanol at 0 to 5℃, stirred for 30 min, and the solvent was concentrated to obtain a viscous crude product. 10 ml of n-heptane was added, and after solid precipitation, stirring was continued for 2 hours, and the solid was drenched with 5 ml of n-heptane. Washing and drying gave 0.5gram ABT199 intermediate 4, TERT-BUTYL 2-((1H-PYRROLO[2,3-B]PYRIDIN-5-YL)OXY)-4-BROMOBENZOATE cas number 1628047-84-6
Thera. Category: Leukemia Drugs
Cas No.: 1628047-84-6
Synonyms: 2-(4-Chlorophenyl)-4,4-dimethyl-1-cyclohexene-1-carbaldehyde;2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-enecarboxaldehyde;2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enecarbaldehyde;4'-chloro-5,5-diMethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-carbaldehyde;2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexene-1-carboxaldehyde;
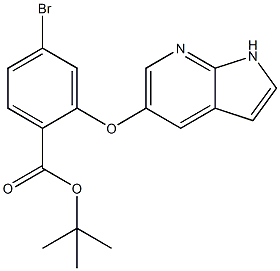
Molecular Formula:C18H17BrN2O3
Molecular Weight: 389.26
Purity: ≥98%
Packing: Export worthy packing
Material Safety Data Sheet: Available on request
|
ABT-199 intermediates
|
|
2-(4-chlorophenyl)-4,4-dimethyl-1-Cyclohexene-1-carboxaldehyde
|
1228837-05-5
|
|
(2-(4-chlorophenyl)-4,4-diMethylcyclohex-1-enyl)Methanol
|
1228780-51-5
|
|
1-[[2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]
methyl]-Piperazine, hydrochloride (1:2)
|
1628047-87-9
|
|
4-Aminotetrahydro-4H-pyran
|
130290-79-8
|
|
3-nitro-4-((tetrahydro-2H-pyran-4-yl)MethylaMino)
benzenesulfonaMide
|
1228779-96-1
|

.png) Contact Now
Contact Now
