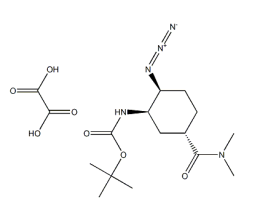
Product Description
Tert-Butyl(1R,2S,5S)-2-azido-5-[(diMethylaMino)carbonyl]cyclohexylcarbaMate oxalic acid is an intermediate of Edoxaban CAS number is 1210348-34-7, molecular formula: C16H27N5O7, Molecular weight: 401.41488, there are three main methods of synthesis:
The first one is the original research company Daiichi Sankyo Company Limited disclosed the synthesis process of cyclohexylamine alcohol through mesylate, sodium azide substitution, hydrolysis, amidation, and hydroamination to obtain intermediate 1. The process yield is not high in the process of mesylate azide, about 30%, and the diastereomeric selectivity is not high. In addition, in order to obtain the dimethylformamide functional group, ethyl ester is required. The steps of hydrolysis, condensation and amidation are relatively tedious, the yield is low, and the cost is high.
Secondly,Daiichi Sankyo Company Limited also discloses a new process for azidation of diformamide-substituted cyclohexylamino mesylate (Reaction Formula 3) in the patent CN101263110 by adding a phase transfer catalyst. The dodecyl chloride pyridine increases the overall yield of the sulfonate to compound 1, but the use of the dangerous reagent sodium azide is still unavoidable.
The existing synthetic processes often have a low azide yield of the ester or amide alignment, a low diastereoselectivity, and a high explosive risk. The industrial production of sodium azide is high, and the conversion step is cumbersome. Insufficient aspect
Step 1: Add 18 kg of acetonitrile to the reaction kettle, add 3.2 kg of t-butanol, cool to -5 to 0 ℃ with ice brine; add 6.1 kg of chlorosulfonyl isocyanate dropwise, and control the temperature between 0 and 5 ℃ during the dropwise addition. After the addition is completed, the mixture is kept warm for about 1 hr; the temperature control is not more than 5 ℃, and 9.2 kg of triethylamine is added dropwise. After the addition is completed, the mixture is kept warm for about 1 to 2 hr;
Step 2: Add 18 kg of water to the reaction kettle, add 4 kg of sodium hydroxide, stir completely dissolved, and cool to -5 to 0 ℃; control temperature does not exceed 5 ℃, and add (1S, 3R, 4R) -3- Amino-4-hydroxy-N,N-dimethylcyclohexylcarboxamide hydrochloride 8 kg; after the addition, stirring for about 30 min; temperature control does not exceed 5 ℃, the Burgess-type reagent obtained in the first step is added to In the reaction solution; after the addition, the reaction is stirred at 0 to 5 ℃ for 3 to 5 hr. After completion of the reaction, the pH of the system was adjusted to 3 to 4 with 6N hydrochloric acid; after the completion of the reaction, 30 kg of ethyl acetate was added to the reaction system, and the mixture was thoroughly stirred; the mixture was allowed to stand, and the mixture was separated, and the aqueous phase was extracted with 30 kg of ethyl acetate. The organic phase was combined; washed once with 20 kg of saturated brine; the organic layer was separated, dried over anhydrous sodium sulfate, filtered.
Step 3: 40 kg of dichloromethane was added to the residue obtained in the second step, 4.0 kg of triethylamine was added, and the mixture was cooled to -5 to 0 ℃ with iced water, the temperature was not more than 10 ℃, and 4.3 kg of methanesulfonyl chloride was added dropwise; After the addition is completed, the heat is reacted at 5 to 10 ℃ for 3 to 5 hr. After the reaction is completed, 50 kg of water is added to the reaction system; the mixture is cooled to -5 to 0 ℃ with iced water, stirred and crystallized for 3 to 5 hr; filtered, rinsed with water, solid is collected, and dried to obtain a white solid dry product of about 9.16. Kg.
Step 4: Adding 9 kg of acetonitrile and 9.15 kg of white solid obtained in the third step to the reaction vessel, adding 2.3 kg of triethylamine; heating the reaction system to 58-62 ℃, maintaining the reaction for 4-5 hr; adding 8.2 kg of pyridine, adding 4kg water; continue to heat to 80 ~ 85 ℃, and then react 4 ~ 5hr; after the reaction is completed, the reaction solution is cooled 10 ~ 20 ℃, with stirring, add 2.5kg of sodium hydroxide and 2.5kg of water formulated solution; fully stirred Separating the solid; filtering, filtering the cake with acetonitrile, collecting the filtrate; concentrating the collected filtrate under reduced pressure to obtain a residue, adding 52 kg of acetonitrile, heating to 30-40 ℃, insoluble matter; pad diatomaceous earth filtration, The filter cake was rinsed with acetonitrile, and the filtrate was collected; 2.65 kg of water was added to the filtrate, and cooled to 10 to 15 ℃; 2.65 kg of oxalic acid dihydrate was added; the temperature was lowered to 0 to 5 ℃, and the mixture was kept warm for about 3 to 5 hr; Solid; dried to give 6.82 kg of edoxaban intermediate Tert-Butyl(1R,2S,5S)-2-azido-5-[(diMethylaMino)carbonyl]cyclohexylcarbaMate oxalic acid CAS 1210348-34-7.(which is also called EthanediaMide iMpurity A)
Thera. Category: Anticoagulant drug
Cas No.: 1210348-34-7
Synonym:tert-Butyl [(1R,2S,5S)-2-aMino-5-[(diMethylaMino)carbonyl]cyclohexyl]carbaMate oxalate(for Edoxaban);tert-butyl N-[(1R,2S,5S)-2-amino-5-(dimethylcarbamoyl)cyclohexyl]carbamate;(1S,2R,4S)-2-[(tert-Butoxycarbonyl)Amino ]-4-[( dimethylamino)carbonyl]- cyclohexyl ammonia oxalate;Tert-Butyl(1R,2S,5S)-2-azido-5-[(diMethylaMino)carbonyl]cyclohexylcarbaMate oxalic acid;oxalic acid tert-butyl N-[(1R,2S,5S)-2-aMino-5-(diMethylcarbaMoyl)cyclohexyl]carbaMate;(2-Azido-5-diMethylcarbaMoyl-cyclohexyl)-carbaMic acid tert-butyl esteroxalic acid;2EthanediaMide iMpurity A;tert-Butyl [(1R,2S,5S)-2-aMino-5-[(diMethylaMino)carbonyl]cyclohexyl]carbaMate oxalate;
Molecular Formula: C16H27N5O7
Molecular Weight: 401.41488
Purity: ≥98.%
Packing:Export worthy packing
Material Safety Data Sheet:Available on requestion
Specification:
|
HPLC
|
≥99%
|
white crystalline powder
|
|
Single impurity
|
≤1%
|
|
enantiomer
|
≤0.5%
|
|
water content
|
≤1%
|
Related product:
Edoxaban (tosylate Monohydrate) 1229194-11-9
Edoxaban 912273-65-5
Edoxaban 480449-70-5
Tert-Butyl(1R,2S,5S)-2-azido-5-[(dimethylamino)carbonyl]cyclohexylcarbamate oxalic acid Cas 1353893-22-7
N-[(1R,2S,5S)-2-amino-5-[(dimethylamino)carbonyl]cyclohexyl]-Carbamic acid, 1,1-dimethylethyl ester, ethanedioate (1:1)CAS 1210348-34-7

.png) Contact Now
Contact Now