Product Description
Bictegravir sodium CAS Number is 1807988-02-8. The FDA approved the Bictegravir sodium combination as a new anti-HIV drug on February 12, 2018. Some professionals expect Bictegravir sodium combination CAS Number 1807988-02-8 has the sales value more than $5 billion in 2022. The drug is the latest development of AIDS drugs by GileadSciences. The company's CEO, John Milligan, believes this will be the best drug ever. It will become the "most important product" in the AIDS market.
Relevant clinical information shows that the first phase of clinical research included 20 adults with chronic HIV infection. Participants were either receiving antiretroviral therapy or were experienced in treatment, but had not used integrase inhibitors before and did not take antiretroviral drugs for at least 12 weeks. Except one people, All were white man, with an average age of 35. At baseline, their mean CD4 count was approximately 440 cells/mm3 and the mean HIV RNA level was 4.4 log10 copies/ml. Participants were randomly assigned to 5, 25, 50, 100 mg of the Bictegravir sodium combination and placebo groups for 10 days on an empty stomach once a day. Drug resistance testing is performed between baseline and maximum dose. On day 11, the Bictegravir sodium combination resulted in a rapid dose-dependent decrease in viral load from -1.45 to -2.43 log10, while there was no change in the placebo group. In all treatment groups of the Bictegravir sodium combination, viral load decreased throughout the treatment period, from 50 mg on day 14 to 100 mg on day 17. At the end of treatment, one participant at a dose of 50 mg and two participants at a dose of 100 mg inhibited the virus below 50 copies/ml. There were no serious adverse reactions in the different dose groups for the side effects of the Bictegravir sodium combination due to side effects.
Thera. Category:Integrase Inhibitor
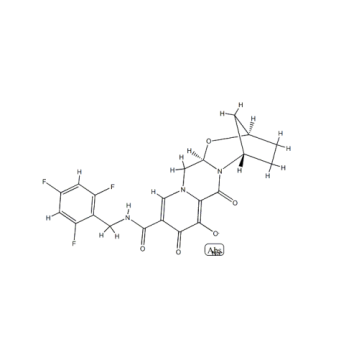
Cas No.:1807988-02-8
Synonyms:Bictegravir;GS-9883; Bictegravir (GS-9883)
MF:C21H17F3N3NaO5
NW:471.36200

Assay: ≥99%
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
