Product Description
Baricitinib phosphate salt CAS number is 1187595-84-1, development code is INCB-28050, it is a JAK inhibitor compound that can be used to treat autoimmune, inflammatory or cancer diseases in which JAK is involved. It is well known that the same drug may have different bioavailability depending on its crystal form. In addition, its stability, fluidity and compressibility may also be different. These physical and chemical properties have certain effects on the application of drugs, thus affecting The efficacy of the drug. Therefore, we need a crystalline form of Baricitinib phosphate salt with superior physiochemical properties, which is more conducive to his use in pharmaceutical processing and pharmaceutical compositions. At present, Baricitinib phosphate salt has three crystal forms, which are A crystal form, H crystal form, and I crystal form.
The preparation process crystal form A of Baricitinib phosphate salt CAS number 1187595-84-1 is as following: weigh 1.0 g of Baricitinib phosphate salt in a sample bottle, add 5 mL of N, N dimethylformamide to make it completely dissolve in the sample bottle. A solvent such as diethyl ether, diisopropyl ether, methyl tert-butyl ether, ethyl acetate or isopropyl acetate was slowly added thereto. After the solid was precipitated from the mixture of the dissolved matter and the organic solvent, an additional 5 mL of an organic solvent was further added dropwise. The resulting mixture was allowed to stand at room temperature overnight, and then the precipitate obtained after standing overnight was filtered and dried in vacuo to give an off-white solid. Such a white solid is the crystalline form A of Baricitinib phosphate salt. The appropriate amount of A crystal form was tested by XRPD in high temperature, high humidity and light. The results showed that Baricitinib phosphate A crystal form had good stability.
The preparation process of Baricitinib Phosphate H crystal form is weighing 1.0 g of Barrickinib phosphate in a sample bottle, add 5 mL of N, N-dimethylformamide to completely dissolve it, and then dissolve in the solution. 20 mL of ethyl acetate at about 3℃ was added thereto, and a solid was immediately precipitated from the mixture of the dissolved matter and the organic solvent, and stirred at 3℃ for 1 hour. The resulting precipitate was filtered and dried in vacuo to give an off-white solid. Such a white solid is the crystalline form of Baricitinib Phosphate H.
The appropriate amount of H crystal form was tested by XRPD in high temperature, high humidity and light. The results showed that Baricitinib phosphate salt crystal form H had good stability.
The preparation process of Baricitinib Phosphate I crystal form is weighing 1.0 g of Baricitinib in a sample bottle, add a mixed solvent of 25 mL of acetonitrile and 8 mL of ethanol, and then raise the temperature to 80℃to makeBaricitinib phosphate salt is dissolved. 4 mL of an ethanol solution containing 3.375 mmol of phosphoric acid was slowly added over 2 minutes, and the solution obtained in this step was further stirred at 80℃ for 2 hours. The solution was then slowly lowered to room temperature over 90 minutes to precipitate a solid. The resulting precipitate was filtered and dried in vacuo to give an off-white solid. Such a white solid is the crystalline form of Baricitinib Phosphate I. The appropriate amount of I crystal form was tested by XRPD in high temperature, high humidity and light. The results showed that Baricitinibphosphate I crystal form had good stability.
Thera Category: JAK Inhibitor
Cas No.: 1187595-84-1
Synonym :Baricitinib phosphate salt;INCB-28050;Baricitinib (phosphate);[1-(Ethylsulfonyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl]acetonitrile phosphate;2-(3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile phosphate
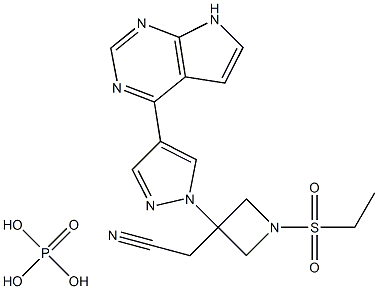
Molecular Formula: C16H20N7O6PS
Molecular Weight: 469.412061
Specifications: Available on request
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Related intermediates:
1) Pyrazole-4-Boronic Acid Pinacol Ester CAS 269410-08-4
2) 1-Boc-3-(Cyanomethylene)Azetidine CAS 1153949-11-1
3) 4-Chloro-5H-pyrrolo[3,2-d]pyrimidine CAS 84905-80-6
4) 4-Chloro-7-((2-(Trimethylsilyl)ethoxy)Methyl)-7H-Pyrrolo[2,3-d]pyrimidine CAS 941685-26-3
5) (4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate CAS 1146629-75-5
6) [4-(1H-Pyrazol-4-yl)-7H-Pyrrolo[2,3-d]pyrimidin-7-yl]methyl Pivalate CAS 1146629-77-7
7) Baricitinib (LY3009104, INCB028050) CAS 1187594-09-7
8) 2-(1-(Ethylsulfonyl)azetidin-3-ylidene)acetonitrile CAS 1187595-85-2

.png) Contact Now
Contact Now
