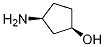Product Description
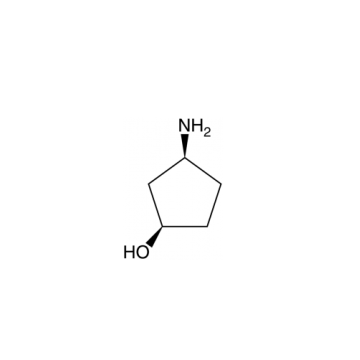
Bictegravir intermediate named (1R,3S)-3-Aminocyclopentanol CAS number is 1110772-05-8. His synthesis process is as follows: (1) Under nitrogen protection, 600 mL of tetrahydrofuran is added to the reaction flask, the stirring device is turned on, and 60.1 gram of carbamide peroxide and 60 g of tert-butyl (1S, 3R)-3-acetylcyclopentylcarbamate are added, control the temperature at 0℃, 138.5 gram of trifluoroacetic anhydride was added dropwise to the obtained mixture. After the addition was completed, the oxidation reaction was carried out for 7 h. After the end of the oxidation reaction by GC, the pH of the obtained system was adjusted with a sodium carbonate solution having a mass concentration of 30%. To 7.5, the temperature was controlled at less than 10℃, stirring was continued for 10 min, and then the layers were allowed to stand. The obtained aqueous phase was extracted with ethyl acetate, and the obtained organic phase was washed successively with 180 mL of a 5% sodium thiosulfate solution.The organic phase is dried over anhydrous sodium sulfate. After filtration, the obtained organic phase is concentrated under reduced pressure to give the corresponding concentrate. The purity of the concentrate is determined by GC. If the purity is greater than 88%, it is used directly to make next step reaction.
(2) Transfer the concentrate obtained in step 1 to a reaction flask, add 300 mL of ethanol, turn on a stirring device, control the temperature to 10℃, add lithium hydroxide aqueous solution dropwise, and carry out hydrolysis reaction at room temperature for 5 h after the addition; GC monitoring reaction, After completion, the obtained system was allowed to stand for stratification, and the obtained aqueous phase was extracted with 240 mL of ethyl acetate. The extraction was repeated once, the organic phase was combined, and the obtained organic phase was washed with 180 mL of brine, The sodium was dried, and after filtration, the obtained organic phase was concentrated under reduced pressure to give white to pale yellow powder.
(3) Under nitrogen protection, 82 g of isopropanol was charged into the reaction flask, the stirring device was turned on, the temperature was controlled at 5℃, 93.4 g of pivaloyl chloride was added dropwise, and the esterification reaction was carried out at 25℃ for 30 min after the completion of the dropwise addition; To the resulting system, a solution of the white to pale yellow powder compound obtained in the step 2 was added dropwise, and the isopropanol solution was prepared 52 g of the white to pale yellow powder compound obtained in the step 2 dissolving in 53 g of isopropanol to make. After the completion of the dropwise addition, the deprotection reaction was carried out for 12 h at room temperature; after the end of the GC monitoring reaction, the temperature of the reaction system was controlled at 0℃ after 1 h, stirred, and the solid was precipitated for 1 h to ensure the solids were analyzed and then under nitrogen protection. Filtration, the obtained filter cake was rinsed with isopropanol at 5℃, and the mixture was depressurized to a drop without dropping, and the filter cake obtained after the leaching was mixed with 40 g of acetone, heated to 50℃ with stirring, and kept under stirring for 2 h; The temperature was lowered to 0℃, filtered under nitrogen atmosphere, and the obtained cake was rinsed with acetone at 5℃, vacuum-dried to a drop-free drop, and then vacuum dried at 40℃ for 12 h to obtain 25.4 g (1R,3S)-3-Aminocyclopentanol hydrochloride CAS 1279032-31-3.
Thera. Category:Integrase Inhibitor
Cas No.:1110772-05-8
Synonyms:Intermediate of Bictegravir;(1R,3S)-3-aminocyclopentanol
MF:C5H11NO
NW:101
Assay: ≥99%
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Usage: Intermediate of Bictegravir

.png) Contact Now
Contact Now