Product Description
There are many reports on the synthesis of crizotinib intermediate (R)-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine CAS number is 877399-00-3. We summed up the main three different ideas and methods.
The first, chiral prolinol induction method. we feel this method has the potential to achieve industrialization, so we will introduce in detail his experimental verification process:
(1) 20.7 g of 2,6-dichloro-3-fluoroacetophenone, 200 ml of tetrahydrofuran, and 2.53 g of (S)-diphenylprolinol are added to a reaction flask, and the mixture is stirred at room temperature for 10 minutes until the solid completely dissolves, using ice-water bath to coo 1℃ and 4.5 g of NaBH4 was added in portions. After the addition is complete, warm to room temperature and stir overnight. TLC showed that the reaction of raw materials was completed. The reaction solution was poured into semi-saturated aqueous ammonium chloride solution, stirred for 30 minutes, extracted with ethyl acetate, and the organic phases were combined. The organic phase was washed with 1N HCl, 5% NaHCO.sub.3 and Saturated salt water, and then dried over anhydrous sulfuric acid. The sodium was dried and concentrated to dryness under reduced pressure for a quantitative yield. The residual light yellow oil was used directly in the next reaction.
(2) The light yellow oil obtained in the previous step is added into a reaction flask, 200 mL of dichloromethane, 15.4 g of DIPEA are added, and the mixture is stirred at room temperature for 10 minutes until the solid is completely dissolved, and cooled to 0℃ in an ice- water bath, and 0.1 mol of methanesulfonyl chloride is added dropwise . After the addition is complete, warm to room temperature and stir overnight. TLC showed the reaction of raw materials was completed, the reaction solution was poured into 1M KHSO4 aqueous solution, stirred for 10min, extracted with ethyl acetate, the organic phase was combined, washed successively with 5% NaHCO3 and saturated salt water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. To dry, get a yellowish solid. Recrystallization with ethyl acetate or n-hexane gave 26.7 g of a white solid.
(3) 25 g of white solid obtained in the previous step was added to the reaction flask, and 2-nitro-3-hydroxypyridine (XII) (14 g), K2CO3 (24 g) and isopropyl acetate (200 mL) were added. Warming reflux reaction for 8h. TLC showed that the reaction of the raw materials was completed. The reaction solution was cooled to room temperature and filtered. The filter cake was washed with 20 mL of isopropyl acetate. The filtrate was washed successively with 1N HCl, 5% NaHCO 3 and saturated salt water, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to give 26.2gram a yellow solid.
(4) The autoclave was charged with 25 g yellow solid obtained in the previous step, nickel (1.5 g, 6 wt%), and 125 mL of ethanol. The air in the kettle was replaced with nitrogen three times, and then the nitrogen in the kettle was replaced with hydrogen three times. The pressure of hydrogen was controlled at 1.5 MPa, and the temperature was raised to 50° C. The reaction was conducted for 4 hours until no hydrogen was absorbed. TLC showed that the reaction of the raw materials was completed. The reaction solution was cooled to room temperature, the catalyst was recovered by filtration, and the filter cake was washed with 10 mL of ethanol. The filtrate was concentrated to dryness under reduced pressure to give 22.5 g of a pale yellow solid.
(5) The reaction flask was added 20 g pale yellow solid of the obtained in previous step, 200 mL of acetonitrile and the solution was cooled to -15°C. 12.3 g of NBS was dissolved in 85 mL of acetonitrile, and the resulting solution was slowly added dropwise to the solution of the pale yellow solid compound obtained in the previous step to maintain the temperature of the reaction solution below -10°C. After the addition, keep warm for 30 minutes. TLC showed that the reaction of the raw materials was completed. The reaction was filtered and the filter cake was washed with cold 10 mL acetonitrile. 200 mL of methylene chloride was added to the filtrate and the temperature was raised to room temperature. Then, sodium thiosulfate KOH aqueous solution was added for washing, the phases were separated, the lower organic layer was washed with water, 5% NaHCO3 solution and saturated slat water, and dried over anhydrous sodium sulfate. Concentrate to dryness under reduced pressure to give a pale yellow solid. The solid was recrystallized from methanol to give 21 g of an off-white solid product. The off-white solid is the target compound.
The Second, 2,6-dichloro-3-fluoroacetophenone was reduced by porcine liver esterase and Mitsunobu reaction, and then reduced by iron powder and NBS treatment to obtain the target compound. The route of this method is long, and the yield of various intermediates will affect the yield of the entire synthesis process.
The third, use S-1-(2,6-dichloro-3-fluorophenyl)ethanol and 2-nitro-3-hydroxypyridine as raw material to form the first step product R-3-(1-( 2,6-Dichloro-3-fluorophenyl)ethoxy)-2-nitropyridine, and then the nitro group is reduced to give R-3-(1-(2,6-dichloro-3-fluorobenzene) (Ethyl)ethoxy)pyridin-2-amine, which is then brominated with the aromatic amine compound to give R-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy) )-Pyridin-2-amine. The biggest drawback of this route is that the starting material S-1-(2,6-dichloro-3-fluorophenyl)ethanol is expensive. If the outsourcing cost is high, it is difficult to make itself, it needs to certain Input.
Thera. Category: Anti-tumor
Cas No.: 877399-00-3
Synonym: (R)-5-BroMo-3-[1-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-pyridin-2-ylaMine;(R)-5-broMo-3-[1-(2,6-dichloro-3-fluoropehnyl)ethoxy]pyridin-2-aMine;InterMediate Ⅰof Crizotinib (R)-5-broMo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-aMine;2-PyridinaMine, 5-broMo-3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-;Crizotinib-H;(R)-5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine;[5-Bromo-3-[(1R)-(2,6-dichloro-3-fluorophenyl)ethoxy]pyridin-2-yl]amine;[5-Bromo-3-[[(R)-1-(2,6-dichloro-3-fluorophenyl)ethyl]oxy]pyridin-2-yl]amine;
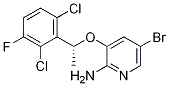
Molecular Weight: 380
Molecular formula: C13H10BrCl2FN2O
Purity: ≥99.%
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
