Product Description
Modafinil intermediate 2-Benzhydrylsulphinylacetic acid CAS number is 63547-24-0, he can also be used to synthesize the refreshment drugs of other central nervous system. With regard to the synthesis of 2-Benzhydrylsulphinylacetic acid CAS 63547-24-0, in a large number of published reports, most of diphenylmethanol and its derivatives are used as starting materials to synthesize 2-diphenylmethylsulfinylacetic acid. These methods have the common characteristics: long reaction path, complex operation of the first-step reaction, low yield, and malodorous products of diphenyl mercaptan, and the synthesis of diphenylmethanol as the starting material is difficult, and the yield is not high. The market price is higher.
One method we feel is more reliable: 1. Using diphenylmethane as a raw material, using the reactivity difference of halogenated alkane reacts with bromine to obtain the first intermediate diphenyl bromide. 2. The condensation reaction of diphenyl bromide with thioglycolic acid provides the second intermediate dibenzothioacetic acid. 3. Dibenzylthioacetic acid is chlorinated to give the third intermediate intermediate diphenylthioacetyl chloride. 4 Dibenzylthioacetyl chloride is amidated to give the target compound 2-diphenylmethylsulfoxide acetic acid. This method uses diphenylmethane, an inexpensive and readily available starting material. After the formation of diphenyl bromide, the reaction method of condensing with thioglycolic acid is lower than the cost of starting the reaction with diphenylmethanol, and the side reaction is reduced. The purification method is simpler and the yield is higher. In the third and fourth step reactions, dibenzylthioacetic acid produces dibenzylthioacetyl chloride followed by amidation, which results in an increase in the yield of the amidation after formation of the ester, simplifying the operation; at the same time, this method Based on the literature, the reaction conditions were optimized. The temperature of the third step of adding thionyl chloride and the fourth step of adding ammonia was changed from the reflux temperature or room temperature to 0 to 5°C, the by-products decreased, and the yield increased. . In general, the cost of this synthesis method is reduced, the reaction conditions are mild, the product yield and quality are relatively high, and it is suitable for industrial production.
Thera. Category: Anti-cancer
Cas No.: 63547-24-0
Synonym: 2-Benzhydrylsulphinylacetic acid;Benzyhydrylsulfinylacetic acid;2-[(Diphenyl-methyl)sulfinyl]acetic Acid;Modafinic Acid;Modafinil Acid;Acetic acid, [(diphenylmethyl)sulfinyl]-;(Diphenylmethylsulfinyl)acetic acid;(Benzhydrylsulfinyl)acetic acid
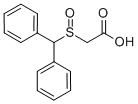
Molecular Weight:274.33486
Molecular formulate:C15H14O3S
Assay: ≥98.%
Appearance: White Crystalline solid
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
