Product Description
Linocaine hydrochloride, CAS number is 6108-05-0, belongs to the class of local anesthetics of amides and it is considered to be an ideal local anesthetic because of its advantages such as rapid, strong, long-lasting, penetrating power, and low cost. Due to the low bioavailability of oral administration, injection or local administration is often used. The existing linocaine hydrochloride gel has a 2% concentration (measured by linocaine hydrochloride monohydrate) and can be used as a local anesthetic. Applied to transurethral examination and treatment requires local anesthesia. However, with the extension of studies on linocaine hydrochloride, it has been found that it can be used for a variety of purposes as local anesthetics , such as in the form of nasal administration for the treatment of migraine. It can also be used as a lubricator for cavitary, which can significantly lubricate the digestive tract and reduce the damage to the lumen when the endoscope enters the body cavity. It can reduce the patient's pain during endoscopy and reduce the esophagus and stomach. The embarrassing situation facilitates smooth inspection under the endoscope.
Local infiltration anesthesia with linocaine hydrochloride is more commonly used in outpatient and partial inpatient surgery. It is loved by most surgeons because of its advantages such as safety, convenience, fast effect, saving time and cost, and facilitating the dynamic evaluation of surgical results during surgery. However, its own shortcomings have also made the popularity of this technology subject to certain restrictions. The obvious shortcomings are the short duration of anesthesia and the inability to resolve intraoperative hemorrhage problems, so it is only suitable for small and superficial surgeries. For long-term, deep-seated surgical procedures that require clear surgical field operations, Because of bleeding and duration of anesthesia,clinical do not use.
The use of linocaine hydrochloride in combination with epinephrine hydrochloride solved the problem of short duration of anesthesia and intraoperative bleeding. The vasoconstrictive effect of epinephrine hydrochloride can make the infiltrative surgical area achieve hemostasis and delay the absorption of local anesthetic drugs, thereby prolonging the anesthetic time. Studies have reported that the combined duration of anesthesia with linocaine hydrochloride and epinephrine hydrochloride is approximately twice that of linocaine hydrochloride alone. The safety of linocaine hydrochloride combined with epinephrine hydrochloride for topical infiltration anesthesia has been confirmed in more studies and no adverse reactions have been found in the clinic. So far there have been no reports of skin and finger necrosis caused by local injections of linocaine hydrochloride and epinephrine hydrochloride. Therefore, a safe dose of epinephrine and linocaine hydrochloride can increase the anesthesia time and achieve the effect of intraoperative hemostasis, which makes up for the shortcomings of linocaine hydrochloride alone.
The safe application of epinephrine hydrochloride CAS number 6108-05-0 combined with linocaine hydrochloride is predicated on safe doses (≤7 mg/kg body weight). Since linocaine hydrochloride and epinephrine hydrochloride have strict usage limits and the concentration of different specifications of products in the clinic is different, the use of linocaine hydrochloride and the patient`s body weight must be accurately calculated to determine the proportions of the two drugs. Drugs of different concentrations are formulated into a mixture of corresponding concentrations. The computational process is cumbersome, it is easy to have calculation errors and there are hidden safety hazards; it wastes valuable medical time; it also makes some doctors give up the use of this technology because of its computational complexity and uncertainty in its calculation process. Therefore, there is an urgent need for a tool that can quickly determine the amount of linocaine hydrochloride and epinephrine hydrochloride required for rapid, accurate, and safe use by simply determining the dose of the injection and the weight of the patient.
Thera. Category: Anesthetic Agents
Cas No.:6108-05-0
Synonym:Diethylamino-2,6-dimethylacetanilide hydrochloride Linocaine hydrochlor;2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide hydrochloride hydrate;
Molecular Structure: 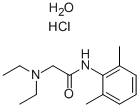
Molecular Formula:C14H25ClN2O2
Molecular Weight:288.81
Pharmacopeia: In house Spec.
Specifications:Available on request
Packing:Export worthy packing
Material Safety Data Sheet:Available on request


.png) Contact Now
Contact Now
