Product Description
Nintedanib Ethanesulfonate Salt CAS number is 656247-18-6 is an oral triple-acting vascular kinase inhibitor developed by Boehringer Ingelheim and was approved by the FDA in October 2014 for the treatment of idiopathic pulmonary fibrosis (IPF), becoming the first tyrosine kinase inhibitor (TKI) approved for the treatment of IPF. Nintedanib Ethanesulfonate Salt has been shown to act on growth factors ,It has potential effects on the pathophysiology of pulmonary fibrosis, the most important of which is the platelet-derived growth factor receptor (PDGFR) Fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR); by blocking these signal transduction pathways involved in fibrosis progression,Nintedanib Ethanesulfonate slowed down by slowing the rate of decline in lung function IPF disease progression. In addition, Nintedanib Ethanesulfonate cas 656247-18-6 has been found to be useful in the treatment of many cancers, including non-small cell lung cancer, prostate cancer, ovarian cancer, and colorectal cancer.
At present, the quality standard of Nintedanib Ethanesulfonate is not included in the domestic and foreign pharmacopoeia, and no reports about the determination of the impurity have been reported at home and abroad. According to the guideline "limit of genotoxic impurities released by the European Pharmacopoeia Administration (EMEA) Principles ", according to Toxicological Toxicity Threshold (TTC) as the threshold for the evaluation of most genotoxic impurities, the maximum limit of genotoxic impurities intake is 1.5μg / g. A daily recommended dose of 300 mg is included in the Nintedanib Ethanesulfonate instruction.
Thera. Category: Anti-cancer
Cas No.: 656247-18-6
Synonym: Nintedanib ethanesulfonate;BIBF 1120 esylate;BIBF-1120 esylate;Nintedanib esylate;Nintedanib ethanesulfonate salt;BIBF1120/nintedanib ethanesulfonate salt;Trinidad Neeb esylate
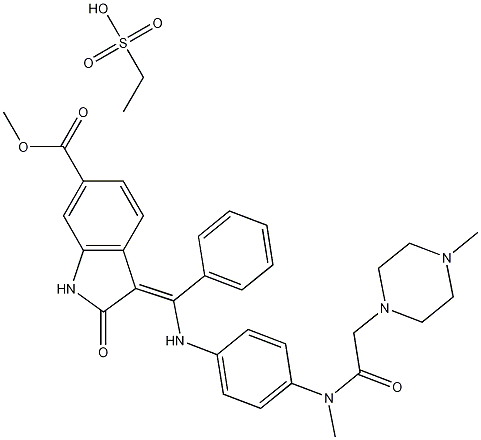
Molecular Weight: 649.75706
Molecular formula: C31H33N5O4.C2H6O3S
Assay: ≥98.%
Appearance: White Crystalline solid
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
